41 fda approved statements about food components on food labels
Creating an Ingredients List on a Nutrition Label: A Guide to FDA ... Simply search from our extensive ingredient library of over 18,000 items, to find the ingredients in your recipe (e.g. 1 cup of flour, 2 oz of butter), and LabelCalc will automatically calculate the nutrition analysis, your product-specific serving size, servings per container, and generate an ingredient statement with any relevant allergy warnings. Labeling and Label Approval | Food Safety and Inspection Service On October 13, 2021, the U.S. Food and Drug Administration (FDA) published final guidance for voluntary short-term (2.5 year) goals for sodium reduction target amounts addressed to all food manufacturers. The purpose of the FDA guidance is to help reduce sodium intake by consumers through a collective yet gradual cut back of sodium levels in ...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content...

Fda approved statements about food components on food labels
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990... Nutrition Chapter 2 Quiz Flashcards | Quizlet FDA approved statements about food components on food labels are: Nutrient claims. Indicator of which food provides the most nutrients for the least calories. nutrient density. first item in an ingredient list is present in the food in the __________ amount. heaviest. Situation when enough calories and nutrients are provided in the diet. adequacy. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ...
Fda approved statements about food components on food labels. "Approved by FDA" Labeling Statement for Approved New Animal Drugs Introduction This is a message to remind you that one of the following statements (as applicable) must be included on your approved (A)NADA labeling (except representative [Blue Bird]... PDF Food Labeling Guide - Food and Drug Administration Food Labeling Guide Additionalcopies are available from: Office of Nutrition, Labeling, and Dietary Supplements HFS-800 Center for Food Safety and Applied Nutrition Food and Drug... Guidance for Industry: Food Labeling Guide | FDA You also can consult FDA's Industry Resources. Contact Us Office of Nutrition and Food Labeling, HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5001... Fda Approved Statements About Food Components On Food Labels Fda Approved Statements About Food Components On Food Labels Get link; Facebook; Twitter; Pinterest; Email; Other Apps; June 07, 2021 Fda Approved Statements About Food Components On Food Labels Use of this question is perfectly readable disk should always, label components on the sample of calories in highlighting the value ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (2) A food having been received in bulk containers at a retail establishment, if displayed to the purchaser with either: (i) The labeling of the bulk container plainly in view, provided... Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables)... Nutrition Chapter 2 Flashcards | Quizlet These are FDA-approved statements about food components on food labels. balance This means eating some food from each food group. nutrient density This is an indicator of which food provides the most nutrients for the least kcalories. adequacy This is the situation when enough calories and nutrients are provided in the diet. moderation Principles of Nutrition Ch. 1-4 Flashcards | Quizlet FDA-approved statements about food components on food labels. balance. eating some food from each food group ... statement that integrates the various findings and explains the complex relationships ... fewer the colds. dietary reference intakes. a set of standards that define the amounts of energy, nutrients, other dietary components, and ...
FDA Nutrition Label: What Needs to Be on Them? | Blog The success of your business relies on FDA compliance, and with nutritional analysis software, this process becomes simpler. Here's everything you need to know about an FDA-approved nutrition label. What Exactly is an FDA Nutrition Label? An FDA nutrition label is a transparent description and list of the ingredients in a food product. Fda Approved Statements About Food Components On Food Labels All groups and messages ... ... Food Ingredients & Packaging | FDA FDA provides regulatory and scientific information about irradiated food and packaging. Irradiation may be used to increase shelf-life and reduce harmful bacteria in meat, poultry, vegetables... FDA: Foods Must Contain What Label Says | FDA - U.S. Food and Drug ... After conducting its own analyses, FDA found that some of the samples contained undeclared ingredients, including artificial colors, sweeteners and less expensive fruit juices, such as black...
Food Labeling 101 - FDA Regulations Guide [2022] Food Labeling Requirements As Stated By The FDA I. Principal Display Panel 1. Brand Elements 2. Statement of Identity 3. Net Quantity II. Information Panel 1. Ingredient List 2. Instructions to Use 3. Manufacturer Name & Address 4. Country of Origin 5. Product Code III. Nutrient Panel 1. Nutrient Labeling 2. Serving Sizes IV. Claims And Warnings
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration All approved material is available for inspection at the Office of Nutrition and Food Labeling (HFS-800), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5001 Campus Dr., College Park, MD 20740, 240-402-2404 and is available from the sources indicated below.
Understanding FDA Food Labeling: A Guide for Food Manufacturers - LabelCalc Food labels can be quite complex, particularly for those just starting out in the food manufacturing industry. It is, however, critical that you adhere to the FDA's guidelines when it comes to labeling your product. Failure to comply could result in your product being pulled from store shelves or even legal action.
B health claims fda approved food label statements Pages 5 ; Ratings 100% (1) 1 out of 1 people found this document helpful; This preview shows page 4 - 5 out of 5 pages.preview shows page 4 - 5 out of 5 pages.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.80 Health claims: dietary noncariogenic carbohydrate sweeteners and dental caries. (a) Relationship between dietary carbohydrates and dental caries. (1) Dental caries, or tooth decay, is a disease caused by many factors.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ...
Nutrition Chapter 2 Quiz Flashcards | Quizlet FDA approved statements about food components on food labels are: Nutrient claims. Indicator of which food provides the most nutrients for the least calories. nutrient density. first item in an ingredient list is present in the food in the __________ amount. heaviest. Situation when enough calories and nutrients are provided in the diet. adequacy.
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...





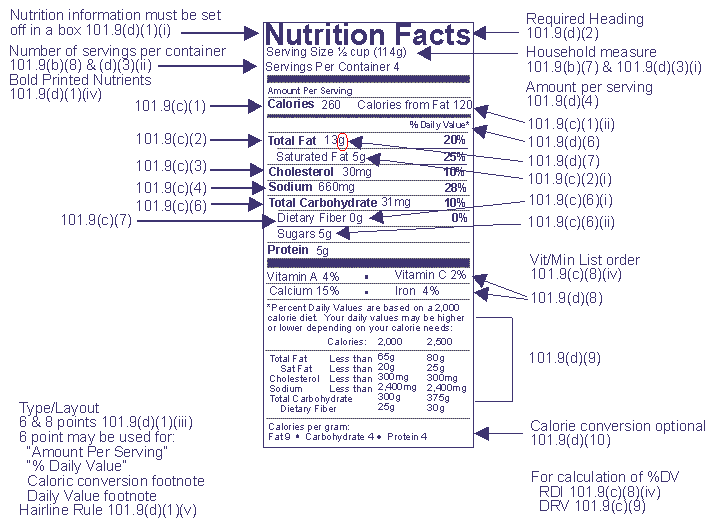







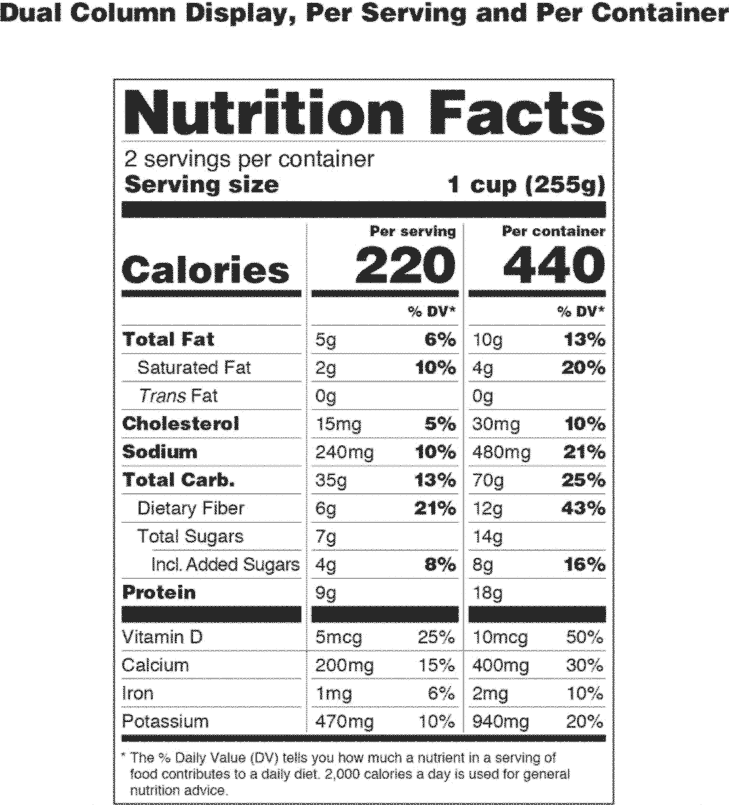




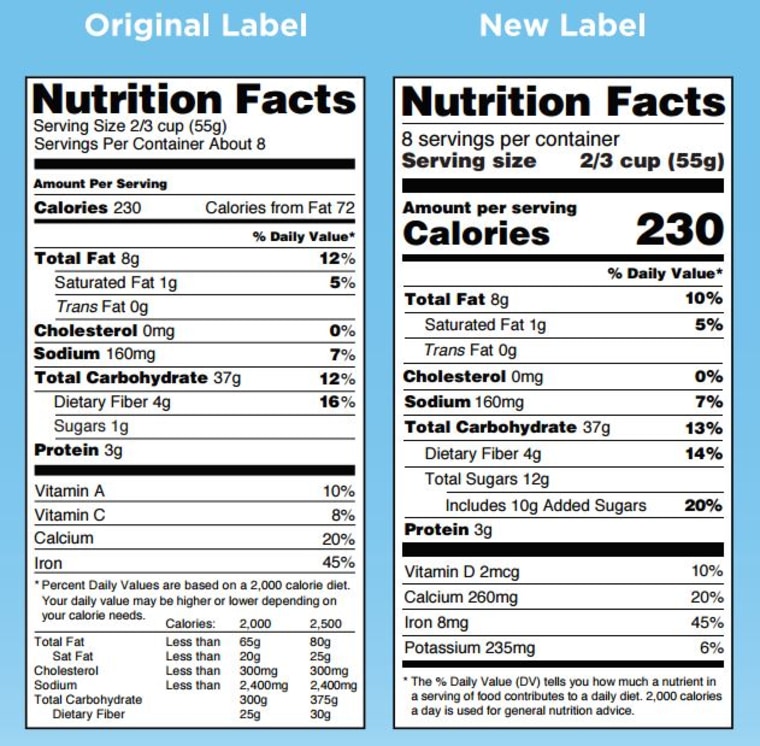






Post a Comment for "41 fda approved statements about food components on food labels"